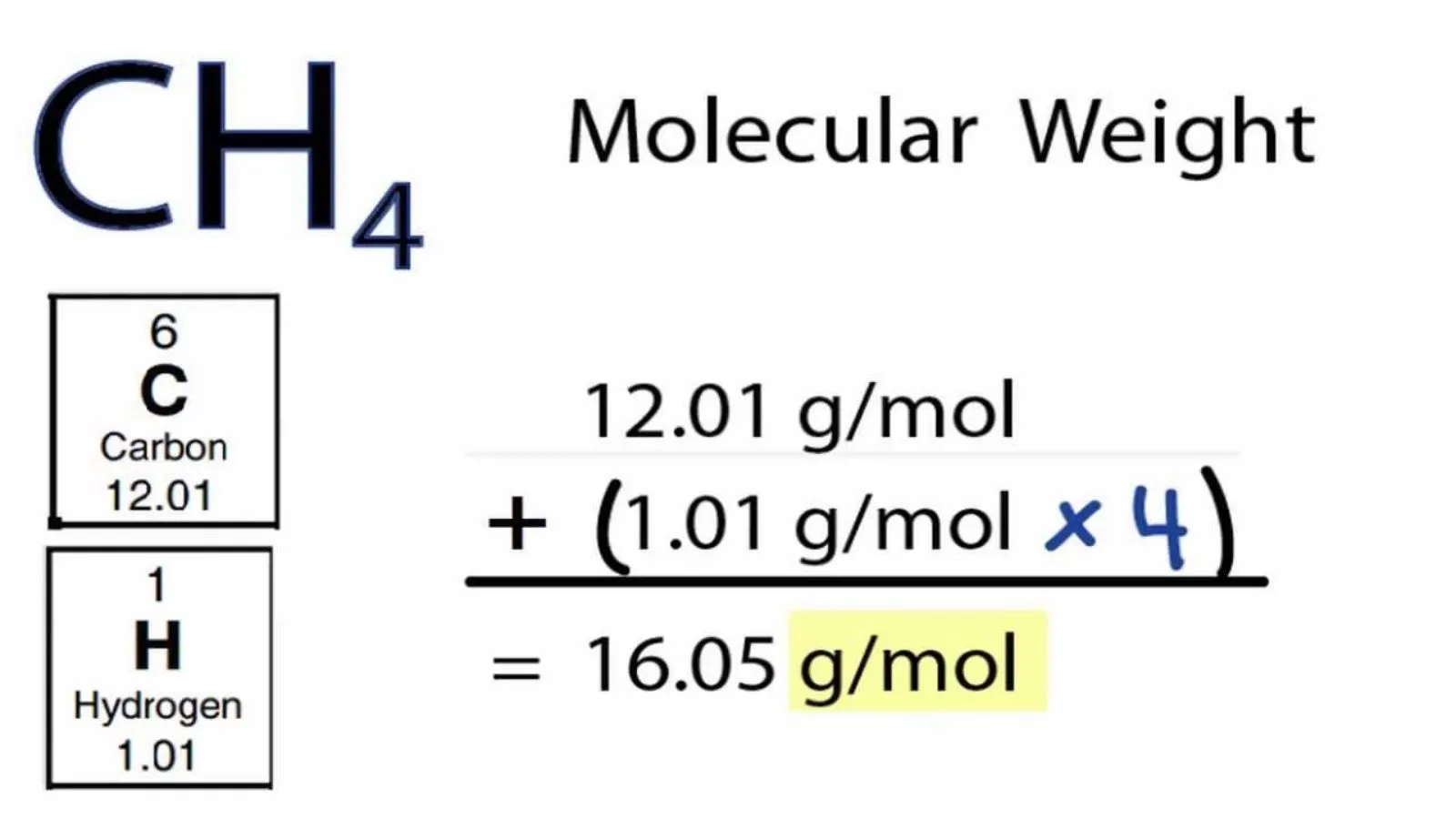
Understanding the molecular weight of a substance is one of the most fundamental concepts in chemistry and biology.
If you are working in a laboratory, studying chemical reactions, or analyzing proteins and polymers, knowing the molecular weight is crucial for accurate calculations and successful experiments.
Molecular weight, also called molar mass, tells us how heavy a single molecule of a substance is and is usually measured in grams per mole (g/mol).
For students, researchers, and professionals alike, determining molecular weight can seem complicated at first, but it becomes straightforward once you understand the methods and formulas.
From simple calculations using the molecular formula to advanced experimental techniques like mass spectrometry and osmometry, there are multiple ways to find the molecular weight of any compound.
In this guide, we will explore all the methods, step-by-step calculations, practical examples, and tips to accurately determine molecular weight for various substances.
What is Molecular Weight?
Molecular weight, also known as molar mass, is the total mass of a single molecule of a substance. It is measured in grams per mole (g/mol) and represents the sum of the atomic weights of all the atoms in the molecule. Molecular weight is essential for understanding chemical reactions, calculating reactants and products, and analyzing substances in both chemistry and biology.
It’s important to note the difference between molecular weight and molecular mass. While molecular weight is a dimensionless quantity calculated relative to the carbon-12 isotope, molecular mass refers to the actual mass of a molecule measured in atomic mass units (amu). For most practical purposes, especially in labs and chemistry calculations, molecular weight and molecular mass can be used interchangeably.
Example 1: Water (H₂O)
- Hydrogen (H) atomic weight = 1 g/mol
- Oxygen (O) atomic weight = 16 g/mol
- Molecular weight = (2 × 1) + (1 × 16) = 18 g/mol
Example 2: Carbon Dioxide (CO₂)
- Carbon (C) atomic weight = 12 g/mol
- Oxygen (O) atomic weight = 16 g/mol
- Molecular weight = 12 + (2 × 16) = 44 g/mol
Molecular weight is not only used for small molecules but also for larger biomolecules like proteins and polymers. Knowing the molecular weight helps scientists calculate concentrations, prepare solutions accurately, and understand the physical and chemical properties of substances.
By understanding molecular weight, you lay the foundation for accurately determining the amount of substances in experiments, making calculations in chemical reactions, and interpreting laboratory data efficiently.
Why Knowing Molecular Weight Matters
Knowing the molecular weight of a substance is essential in many scientific and practical applications. In chemistry, it allows researchers to accurately calculate how much of each substance is needed in a reaction. This is crucial for stoichiometry, where the proportions of reactants determine whether a chemical reaction proceeds efficiently and safely. Using incorrect molecular weights can lead to inaccurate measurements, wasted materials, or failed experiments.
In pharmaceuticals, molecular weight plays a key role in drug formulation and dosing. For example, the effectiveness of a medication often depends on its precise molecular concentration. Understanding the molecular weight helps chemists prepare solutions at exact concentrations to ensure proper efficacy and safety.
In biochemistry, molecular weight is important for analyzing proteins, enzymes, and DNA. Techniques like gel electrophoresis or mass spectrometry often rely on knowing the molecular weight to identify or quantify biomolecules. For instance, determining the molecular weight of a protein can reveal its structure and function in the body.
Even in materials science and polymer chemistry, molecular weight affects properties such as strength, flexibility, and melting point. Polymers with different molecular weights behave differently, so accurate determination is critical for creating materials with desired characteristics.
Ultimately, understanding molecular weight provides the foundation for accurate measurements, safer experiments, and reliable research outcomes. Whether you are a student, researcher, or professional, knowing the molecular weight ensures precision in all scientific work.
Methods to Determine Molecular Weight
There are several methods to determine molecular weight, broadly classified into theoretical calculations and experimental techniques. Choosing the right method depends on the type of substance, its size, and the level of accuracy required.
1. Theoretical Method – Calculation from Molecular Formula
This is the simplest method and works well for small molecules. To calculate molecular weight, you sum the atomic weights of all atoms in the molecule. For example, glucose (C₆H₁₂O₆) has a molecular weight of:
- Carbon (C) = 12 × 6 = 72
- Hydrogen (H) = 1 × 12 = 12
- Oxygen (O) = 16 × 6 = 96
- Total molecular weight = 72 + 12 + 96 = 180 g/mol
This method is straightforward, inexpensive, and highly accurate for known molecular formulas.
2. Experimental Methods
For large molecules like proteins or polymers, experimental techniques are necessary:
- Mass Spectrometry (MS): Measures the mass-to-charge ratio of ions to determine molecular weight accurately.
- Osmometry: Uses osmotic pressure to estimate the molecular weight of polymers in solution.
- Ultracentrifugation: Separates molecules based on size and mass to calculate molecular weight.
- Gel Permeation Chromatography (GPC): Measures molecular weight distribution in polymers.
Each method has its advantages and limitations. While theoretical calculations are fast and simple, experimental methods are essential when the molecular formula is unknown or for large, complex molecules.
By understanding these methods, scientists and students can choose the most suitable approach for accurate molecular weight determination.
Step-by-Step Guide to Calculating Molecular Weight
Calculating molecular weight from a chemical formula is a straightforward process if you follow these steps carefully. This method is especially useful for small to medium-sized molecules where the chemical formula is known.
Step 1: Identify All Atoms in the Molecule
Start by writing down the molecular formula of the substance. Count how many of each type of atom is present. For example, for glucose (C₆H₁₂O₆), there are 6 carbon (C) atoms, 12 hydrogen (H) atoms, and 6 oxygen (O) atoms.
Step 2: Find Atomic Weights
Refer to the periodic table to get the atomic weights of each element. Hydrogen is 1 g/mol, carbon is 12 g/mol, and oxygen is 16 g/mol.
Multiply Atom Counts by Atomic Weights
Multiply the number of each atom by its atomic weight:
- Carbon: 6 × 12 = 72
- Hydrogen: 12 × 1 = 12
- Oxygen: 6 × 16 = 96
Add the Totals
Sum all the values to get the total molecular weight: 72 + 12 + 96 = 180 g/mol
Check Your Work
Double-check atom counts and arithmetic to avoid errors. Mistakes in counting atoms or using incorrect atomic weights are common and can affect experimental calculations.
This step-by-step method can be applied to any molecule with a known formula. It’s a simple, cost-free, and reliable way to determine molecular weight, making it an essential skill for students, chemists, and researchers alike.
Mass Spectrometry: A Practical Approach
Mass spectrometry (MS) is one of the most precise and widely used methods for determining molecular weight, especially for complex or large molecules like proteins, peptides, and polymers. This technique measures the mass-to-charge ratio (m/z) of ionized molecules, providing an accurate molecular weight that is often difficult to obtain using calculations alone.
How It Works:
- Ionization: The sample molecules are converted into charged ions. Common ionization methods include Electrospray Ionization (ESI) and Matrix-Assisted Laser Desorption/Ionization (MALDI).
- Acceleration and Deflection: These ions are accelerated in an electric or magnetic field, causing them to travel at speeds determined by their mass-to-charge ratio.
- Detection: A detector records the time or position where the ions hit, which is then used to calculate their molecular weight.
Advantages of Mass Spectrometry:
- Extremely accurate even for large and complex molecules.
- Can detect impurities or fragments in a sample.
- Useful for identifying unknown compounds.
Example: Determining the molecular weight of a small protein like insulin (~5800 g/mol) is challenging using simple calculations due to its multiple amino acids. Mass spectrometry allows precise determination by analyzing the ions generated from the protein.
While MS requires specialized equipment and expertise, it is invaluable in biochemistry, pharmaceutical research, and analytical chemistry. By combining speed, accuracy, and sensitivity, mass spectrometry provides reliable molecular weight data for both research and industrial applications.
Osmometry and Other Experimental Techniques
In addition to mass spectrometry, other experimental methods such as osmometry, ultracentrifugation, and gel permeation chromatography (GPC) are commonly used to determine molecular weight, particularly for polymers and large biomolecules. These methods are especially useful when the molecular formula is unknown or when studying complex mixtures.
Osmometry measures the osmotic pressure of a solution. When a polymer or solute is dissolved in a solvent, the osmotic pressure depends on the number of molecules present. By applying the van’t Hoff equation, scientists can calculate the molecular weight of the solute. Osmometry is widely used in polymer chemistry and protein studies because it is relatively simple and does not require sophisticated equipment.
Ultracentrifugation separates molecules based on their size and density by spinning samples at extremely high speeds. The sedimentation rate of molecules allows researchers to calculate their molecular weight. This method is particularly effective for proteins and nucleic acids.
Gel Permeation Chromatography (GPC), also known as size-exclusion chromatography, separates molecules based on size. Larger molecules pass through a porous gel faster than smaller ones. By comparing the elution time of unknown molecules to standards of known molecular weight, scientists can determine molecular weight distributions in polymers and other macromolecules.
Each of these experimental techniques has advantages and limitations. While osmometry is simple, ultracentrifugation and GPC provide more detailed information about molecular weight distributions. Combining these methods with mass spectrometry allows for accurate determination of molecular weight, even in complex samples.
Factors Affecting Molecular Weight Determination
Determining molecular weight accurately depends on careful measurement and proper technique. Several factors can affect the results, and understanding these is essential for reliable data.
1. Sample Purity:
Impurities in a sample can significantly alter molecular weight calculations. Even small amounts of contaminants can skew experimental results, especially in techniques like osmometry or mass spectrometry. It’s important to use highly purified compounds whenever possible.
2. Temperature and Pressure:
Experimental methods, particularly osmometry, are sensitive to temperature and pressure. Changes in these conditions can affect the behavior of molecules in solution, leading to inaccurate molecular weight measurements. Maintaining controlled lab conditions is crucial for consistency.
3. Instrument Calibration:
All experimental instruments, including mass spectrometers, ultracentrifuges, and chromatographs, require proper calibration. Incorrect calibration can lead to systematic errors and inaccurate molecular weight determinations. Regular maintenance and calibration checks are essential.
4. Isotopic Variations:
Some elements exist naturally as mixtures of isotopes, which can slightly affect molecular weight calculations. For example, chlorine exists as ³⁵Cl and ³⁷Cl. Considering isotopic distributions is important in high-precision measurements, especially for large or halogen-containing molecules.
5. Measurement Technique:
Different techniques have inherent limitations. For instance, osmometry provides average molecular weight but not distribution, while GPC gives distribution but may require standards. Selecting the appropriate method for your sample type ensures accurate results.
Applications of Molecular Weight in Real Life
Molecular weight plays a crucial role in many scientific, industrial, and medical applications. Understanding the weight of molecules allows chemists, biologists, and engineers to work more precisely and efficiently.
1. Pharmaceutical Industry:
In drug development, molecular weight determines dosage, absorption, and distribution of medicines in the body. Accurate molecular weight calculations ensure proper formulation of tablets, injections, and solutions, making drugs safe and effective. For example, the molecular weight of insulin or antibiotics is critical for preparing precise doses.
2. Chemical Reactions:
Chemists rely on molecular weight to calculate the amount of reactants and products in reactions. Stoichiometry depends on knowing exact molecular weights to achieve desired chemical yields. Using incorrect weights can result in failed experiments or wasted materials.
3. Biochemistry and Molecular Biology:
Molecular weight is essential in studying proteins, DNA, and enzymes. Techniques like gel electrophoresis, ultracentrifugation, and mass spectrometry use molecular weight to identify or quantify biomolecules. For instance, determining the molecular weight of a protein can reveal its structure and biological function.
4. Polymer and Material Science:
In polymers, molecular weight affects strength, flexibility, viscosity, and melting point. Materials engineers use molecular weight data to design plastics, fibers, and coatings with desired physical properties.
5. Industrial Applications:
Molecular weight information is also used in food chemistry, environmental testing, and nanotechnology to develop better products and ensure safety standards.
Common Mistakes to Avoid
Determining molecular weight may seem straightforward, but there are several common mistakes that can lead to inaccurate results. Being aware of these pitfalls can save time, resources, and ensure precise calculations.
1. Miscounting Atoms in the Formula:
One of the most frequent errors is incorrectly counting the number of atoms in a molecular formula. For example, confusing H₂O₂ with H₂O will produce entirely different molecular weights. Always double-check the molecular formula before starting calculations.
2. Using Incorrect Atomic Weights:
Atomic weights are often rounded, but using incorrect or outdated values can cause errors, especially in precise experiments. Always refer to the latest periodic table and use standard atomic weights for calculations.
3. Ignoring Isotopic Variations:
Some elements, like chlorine or bromine, exist naturally as mixtures of isotopes. Ignoring isotopic distributions can slightly affect molecular weight calculations, particularly for high-precision measurements.
4. Confusing Molecular Weight with Molecular Mass:
Although often used interchangeably, molecular weight is dimensionless relative to carbon-12, while molecular mass is measured in atomic mass units (amu). Using the wrong concept in calculations can lead to errors in lab work.
5. Sample Impurities:
Experimental methods like osmometry or mass spectrometry can be affected by impurities. Impure samples produce skewed molecular weight readings, so purification is critical.
6. Instrumental and Calculation Errors:
Incorrect calibration of instruments, rounding errors, or arithmetic mistakes during calculations are common issues. Carefully checking your work and repeating experiments can reduce errors.
FAQ
1. What is the difference between molecular weight and molecular mass?
Molecular weight is a dimensionless quantity calculated relative to the carbon-12 isotope, while molecular mass refers to the actual mass of a molecule in atomic mass units (amu). For most lab calculations, the terms are used interchangeably.
2. Can I calculate molecular weight without experimental methods?
Yes! For small molecules with a known chemical formula, molecular weight can be calculated directly by summing the atomic weights of all atoms in the molecule.
3. Which method is most accurate for large biomolecules?
For large proteins, polymers, or unknown compounds, mass spectrometry is the most precise. Other methods like osmometry, ultracentrifugation, and gel permeation chromatography are also used depending on the sample type.
4. Why do molecular weights sometimes differ in experiments?
Differences can occur due to sample impurities, temperature or pressure variations, instrument calibration errors, or isotopic variations. Controlling these factors improves accuracy.
5. Is molecular weight important in pharmaceuticals?
Absolutely. Molecular weight is critical for drug dosing, formulation, and absorption. Knowing the exact molecular weight ensures medicines are safe and effective.
6. How do I handle molecules with isotopes?
For molecules with naturally occurring isotopes, consider the average atomic weights or, for precise studies, account for isotopic distributions.
7. Can polymers’ molecular weight be calculated theoretically?
Not easily. Polymers vary in chain length, so experimental methods like GPC or osmometry are usually required.
Conclusion
Determining molecular weight is a fundamental skill in chemistry, biology, and materials science.
If you are a student, researcher, or industry professional, knowing the molecular weight of a substance is essential for accurate calculations, reliable experiments, and successful applications.
Molecular weight, or molar mass, allows scientists to calculate the correct amounts of reactants in chemical reactions, formulate drugs safely, analyze proteins and polymers, and develop new materials with desired properties.
There are multiple ways to determine molecular weight. Theoretical calculations from molecular formulas provide a fast and simple method for small molecules.
For larger or unknown molecules, experimental techniques such as mass spectrometry, osmometry, ultracentrifugation, and gel permeation chromatography are more appropriate.
Each method has its advantages and limitations, and the choice depends on the type of molecule, the sample purity, and the desired level of accuracy.
Accuracy is critical, and several factors can affect molecular weight determination, including sample impurities, isotopic variations, instrument calibration, and environmental conditions.
Being aware of these factors and avoiding common mistakes ensures reliable results.
In real-life applications, molecular weight impacts drug development, chemical reactions, protein analysis, polymer manufacturing, and industrial processes.
Understanding both theoretical and experimental methods equips scientists and students with the tools to work effectively in laboratories and research settings.

I’m Robert Silva, a quotes expert at Quotesfuel.com — delivering powerful words and daily inspiration to keep your spirit fueled!